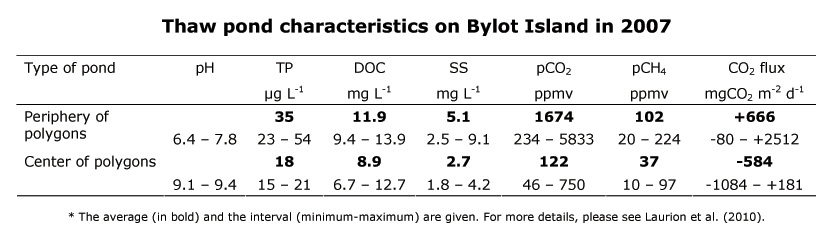
The following table presents pH values, total phosphorus concentrations (TP), dissolved organic carbon (DOC), suspended solids (SS - a turbidity indicator), partial pressures of CO2 and CH4 (for comparison, atmospheric values are, overall on the planet, 380 ppmv for CO2 and 1.7 ppmv for CH4), as well as estimates of CO2 flux between the water and the atmosphere, thus summarizing the principal characteristics of Bylot Island ponds, as measured in 2007.
|
Certain similarities exist between the ponds formed on the center and the periphery of polygons, but also many noteworthy differences. Both types of ponds are relatively rich in nutrients and present greater biological activity in comparison with arctic lakes of the region: estimates range from 10 to 50 times more active in the summer. Ponds in the center of polygons have a more alkaline pH, due to the high photosynthetic activity of cyanobacteria (thick algal formations in bottoms of ponds that feed zooplankton). Ponds on the periphery of polygons are more turbid and contain a greater quantity of phosphorus and dissolved organic carbon. Further, the dissolved organic carbon in these ponds is more colored (data not presented in the table), which greatly reduces the amount of light reaching bottom, and consequently reduces both algal photosynthetic activity and water temperature. This disparity between the two types of ponds is due to peripheral ponds being exposed to a much more active erosive process, increasing their turbidity and decreasing their transparency to incident light.
Both types of thaw ponds are supersaturated with CH4 in summer: the water concentration is greater than the air. This is because of microbial respiration, especially that at the bottom of the ponds, where oxygen is rare. Peripheral ponds are also supersaturated with CO2 in most cases during summer, again due to microbial respiration, but in this case in the presence of oxygen. However, the ponds in the centers of the polygons are under-saturated in CO2: concentration in the water is less than that in the air. This under-saturation is caused by the elevated photosynthetic activity of cyanobacterial mats (see Microbial life below), which uses up the CO2 in the same way as plants and trees. Photolysis of dissolved organic carbon (see below) is another phenomenon that can generate CO2 in water. This non-biological process is caused solely by the sun radiation (in particular ultraviolet rays) and brings about a decomposition of complex molecules into smaller molecules, including CO2.

And so, this begs the question: are these thaw ponds sinks or sources of greenhouse gas? And which carbon source is used by the microbes that produce greenhouse gas? Climate implications are entirely different, depending on whether the carbon comes from that previously stored in tundra soil, or if it comes from carbon recently fixed by plants in the catchment.
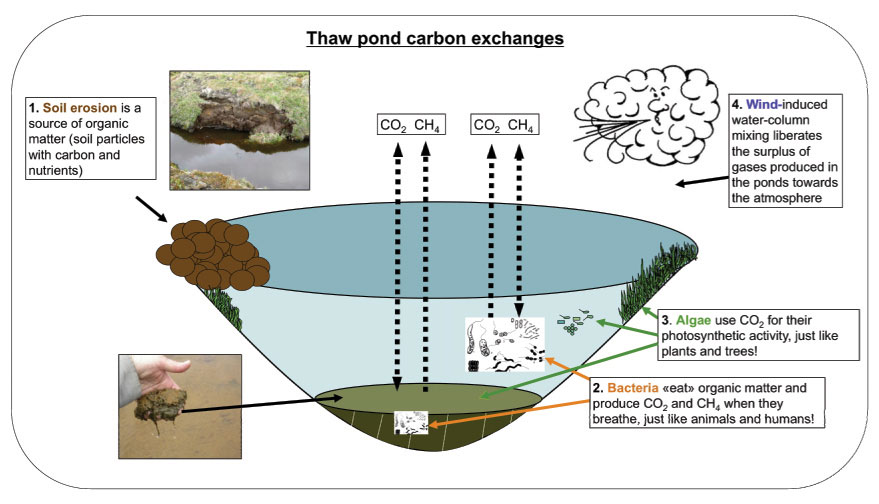
As mentioned earlier, there are processes that increase greenhouse gas in the water and other that generate losses of greenhouse gaz. Gains are microbial respiration (which generates CO2 directly, or initially CH4, which can then be oxidized as CO2) and photolysis of dissolved organic carbon (which generates CO2). Losses are photosynthesis, which transforms CO2 into oxygen, and methanotrophy, which oxidizes CH4 into CO2. Gas concentrations at a given time will express the sum of the gains and losses of the gas in question. When a gas is in excess in a body of water, it has a tendency to equilibrate with the air above (by diffusion), which creates a positive flux towards the atmosphere (positive flux values in the preceding table), and vice versa. These fluxes are however greatly increased by wind, which causes turbulence and mixing of water: the greater the wind speed, the greater the fluxes (if gains are high enough). Greenhouse gas fluxes can be measured using a floating chamber attached to an infrared gas analyzer.
Results so far suggest that while the ponds in the center of the polygons are CO2 sinks, they remain CH4 sources, whereas ponds on the periphery are sources for both CO2 and CH4.
|
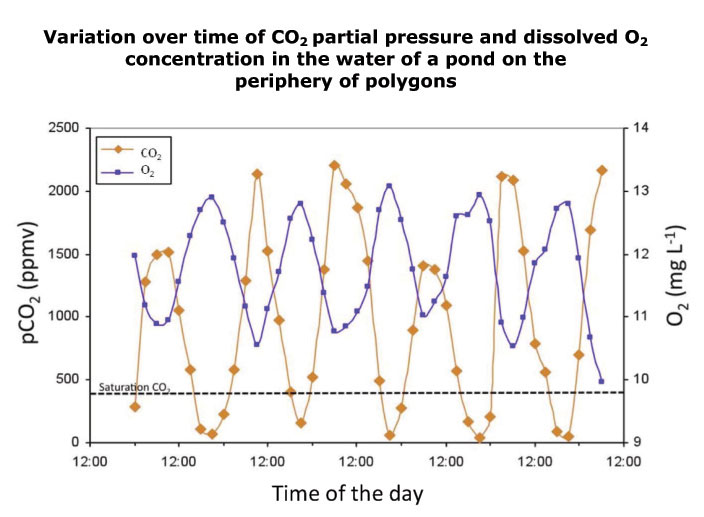
However, depending on the specific biological characteristics of certain ponds (for example, the composition of microbial and algal community, which varies according to the pond's physicochemical properties), a CO2 flux inversion can be observed within the same day. We saw this in a peripheral pond that had been colonized by aquatic plants instead of a cyanobacterial mat: photosynthetic activity and microbial respiration influenced gas concentrations and followed a diurnal cycle (see figure below). Even when under-saturation of CO2 occurred (at the end of the day, where the curve crosses the dashed bar which approximately corresponds to CO2 saturation of the water), most of the time the pond is supersaturated and overall remains a carbon source to the atmosphere. In parallel, we observed fluctuations of dissolved oxygen in the water, resulting from aquatic plant activity.
|
Microbial life
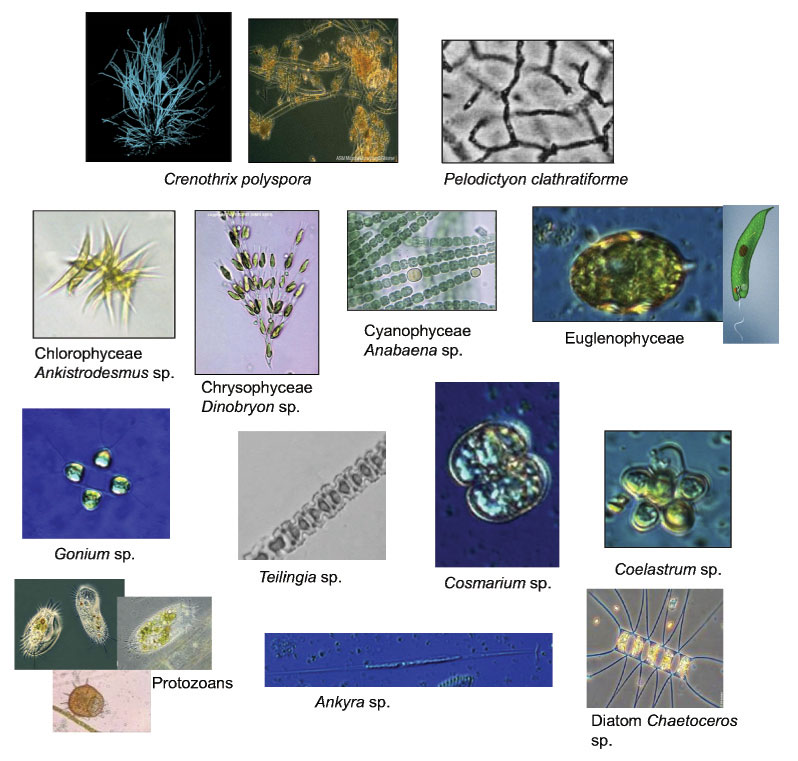
Microbial life flourishes in Bylot Island ponds. These microbes, measuring from 1 to about 100 micrometers (1 micrometer = 1 thousandth of a millimeter), have been identified by molecular biology approaches and by microscopy. Here are some examples, seen through a microscope:
|
 | |
| Dry thaw ponds with their mats or orange cyanobacteria, with a galcial river on the left, erosion of the peat layer. Close up of a portion of cyanobacterial mat. |
These ponds offer a relatively welcoming environment for very primitive microorganisms, such as Bacteria and Archaea, as well as for others, more evolved, such as green and golden algae (including, for example, diatoms). There are methanogens (microorganisms which produce CH4) among the Archaea, whilst among the Bacteria, there are methanotrophs (which consume CH4) and cyanobacteria. These latter form mats on pond bottoms and are responsible for significant photosynthetic activity. On the surface, these mats appear orange-colored, due to the presence of photoprotective pigments (carotenoids), while underneath they are green. These microorganisms need water, light, nutrients and a carbon source for respiration and for photosynthesis. They are, however, very resistant: when the pond dries, their activity is simply interrupted until favorable conditions return. The make-up of the pond microbial assemblages influences the balance between the processes that generate and those that consume greenhouse gas.
Photolysis of dissolved organic carbon
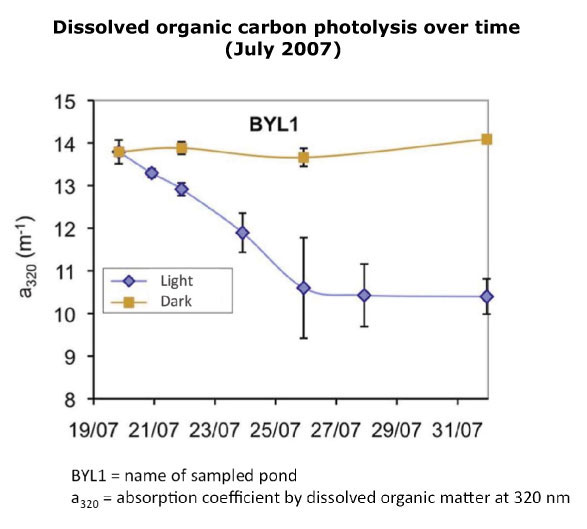
Photolysis is the process of chemical decomposition by light of dissolved organic carbon into smaller molecules (including CO2). Consequently, photolysis is of greater importance in shallow ponds with little turbidity, as they have greater exposition to sunlight. The absorption coefficient of light by water at a wavelength of 320 nm (1 nm = 1 thousandth of a micrometer), named a320, is used to measure the colored fraction of dissolved organic carbon (that which is broken down by light). Photolysis is measured with the help of a spectrophotometer, by examining the decrease of a320 over time as sunlight is having its effect.
Water samples taken in a pond in July 2007 were filtered to remove microorganisms and thus observe only the effect of light. Then these samples were exposed to light or darkness (controlled conditions) before their examination with the spectrophotometer. The results demonstrate how the breakdown of organic molecules by light is expressed by a decrease of watercolor. It would seem that part of the molecules that give to water its color are transformed into CO2, thus contributing to the production of greenhouse gaz. This fraction remains unknown. Other than CO2, photoproducts can be smaller organic molecules, which microorganisms can use more easily (increasingly labile molecules). Thus, one indirect effect of dissolved organic carbon photolysis is to stimulate microbial activity, which in turn frees up carbon by respiration.
|
| Arctic ponds | Implications for climate change |